Configurable ArmsCustom Robotic Arms
Custom Robotic Arms - Built For Applications
When off-the-shelf is not enough, we build exactly what your workflow needs.
From feasibility to clinical-grade prototypes and pilot production, our team designs and manufactures custom robotic arms and subsystems for medical and assistive applications.
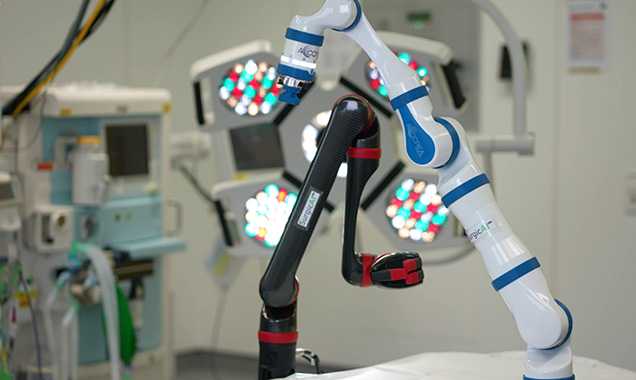
At a Glance
- •Application-driven design: payload, reach, materials, and interfaces engineered to your use case.
- •Medical-environment focus: cleanable geometry, draping strategy, hygienic cable routing.
- •Safety by design: force/speed limits, no-go zones, quick-release mechanics, redundant sensing.
- •Open control: SDK/APIs, optional ROS nodes, logging and test hooks.

What we build
- •Surgical assistance arms (camera, retraction, tool guidance)
- •Imaging & probe-handling manipulators (e.g., ultrasound)
- •Rehab/assistive arms and end-effectors
- •Device-specific motion subsystems (positioners, stages, smart mounts

Engagement model
- •Feasibility & concept stage: requirements, risk review, concept options, early fixtures
- •Alpha prototype: mechanics, control stack, safety envelopes, demo rig.
- •Clinical-grade beta: verification plan, draping, documentation set
- •Pilot manufacture: tooling, test plans, service model, scaling options

Safety & regulatory support
- •Processes aligned with ISO 14971 (risk management) and IEC 60601/62304 expectations.
- •Usability engineering inputs (clinical workflows, HF considerations).
- •Documentation kits (DHF structure, verification templates).
Integration & Compatibility
- •Mounting to tables, carts, rails using custom baseplates
- •Electrical & software interfaces (Ethernet/IP, discrete I/O, pedals, external sensors)
- •Media flanges/end-effectors tailored to your instrument

Options & accessories
- •Vision, ultrasound, or navigation integration kits
- •Quick-swap end-effector ecosystem
- •Data logging & analytics package for testing
Resources & next steps
- •Capabilities deck (PDF)
- •Case studies & references
- •Book a scoping workshop
FAQ
Can you adapt an existing ACCREA platform to our needs?
Yes, many projects start from ARIA-MED or AVROS building blocks.
What if we need unique materials or have some sterilization constraints?
We will design specific protocol to address your cleaning and draping requirements.
Do you ship worldwide?
We support international OEMs; availability and timelines vary by project
Notes & Compliance
- •Availability, features, and intended use may vary by region.
- •ACCREA supplies platforms and components; final device certification is the OEM's responsibility (we can support the process).
- •Always follow clinical judgment, hospital protocols, and applicable regulations.
We Care About Your Privacy
ACCREA Medical Robotics uses cookies to improve and customize users experience on our website. By selecting 'Accept', you consent to the use of all cookies that gather and use information about your interactions with our site to provide personalized content and enhance your digital experience. Please read our Privacy Policy for more information.